What Is The Differences Between The Makeup And Structure Of Salt And Polyester?

Ester group (blue) which defines polyesters.

Close-upward of a polyester shirt

SEM motion picture of a curve in a loftier-surface area polyester fiber with a vii-lobed cross department
Polyester is a category of polymers that contain the ester functional grouping in every repeat unit of their main chain.[1] As a specific fabric, it nearly commonly refers to a type called polyethylene terephthalate (PET). Polyesters include naturally occurring chemicals, such as in plants and insects, as well every bit synthetics such every bit polybutyrate. Natural polyesters and a few synthetic ones are biodegradable, but most synthetic polyesters are not. Constructed polyesters are used extensively in vesture.
Polyester fibers are sometimes spun together with natural fibers to produce a cloth with blended properties. Cotton-polyester blends can exist strong, wrinkle- and tear-resistant, and reduce shrinking. Synthetic fibers using polyester have high h2o, wind and environmental resistance compared to found-derived fibers. They are less fire-resistant and can melt when ignited.[2]
Liquid crystalline polyesters are among the first industrially used liquid crystal polymers. They are used for their mechanical backdrop and heat-resistance. These traits are as well important in their application equally an abradable seal in jet engines.[3]
Types [edit]

A drop of water on a h2o resistant polyester
Polyesters are one of the most economically important classes of polymers, driven specially by PET, which is counted among the article plastics; in 2000 around xxx million tons were produced worldwide.[4] The multifariousness of structures and backdrop in the polyester family is very big, depending on the nature of the R grouping (see outset effigy with blue ester grouping).[1]
Natural [edit]
Polyesters occurring in nature include the cutin component of plant cuticles, which consists of omega hydroxy acids and their derivatives, interlinked via ester bonds, forming polyester polymers of indeterminate size. Polyesters are besides produced by bees in the genus Colletes, which secrete a cellophane-like polyester lining for their cloak-and-dagger breed cells[5] earning them the nickname "polyester bees".[6]
Constructed [edit]
The family of synthetic polyesters comprises:[1]
- Linear aliphatic loftier molecular weight polyesters (Thoun >10,000) are depression-melting (m. p. 40 – eighty °C) semicrystalline polymers and exhibit relatively poor mechanical properties. Their inherent degradability, resulting from their hydrolytic instability, makes them suitable for applications where a possible ecology bear upon is a business organization, e.one thousand. packaging, disposable items or agricultural mulch films[7] or in biomedical and pharmaceutical applications.[viii]
- Aliphatic linear low-molar-mass (Mnorth < 10,000) hydroxy-terminated polyesters are used as macromonomers for the production of polyurethanes.
- hyperbranched polyesters are used as rheology modifiers in thermoplastics or as crosslinkers in coatings[9] due to their peculiarly depression viscosity, proficient solubility and high functionality[10]
- Aliphatic–aromatic polyesters, including poly(ethylene terephthalate) and poly(butylene terephthalate), are high-melting semicrystalline materials (g. p. 160–280 °C) that and accept found use as technology thermoplastics, fibers and films.
- Wholly aromatic linear copolyesters present superior mechanical properties and heat resistance and are used in a number of high-operation applications.
- Unsaturated polyesters are produced from multifunctional alcohols and unsaturated dibasic acids and are cross-linked thereafter; they are used as matrices in composite materials. Alkyd resins are made from polyfunctional alcohols and fat acids and are used widely in the coating and composite industries as they tin can be cross-linked in the presence of oxygen. Also rubber-like polyesters be, called thermoplastic polyester elastomers (ester TPEs). Unsaturated polyesters (UPR) are thermosetting resins. They are used in the liquid country every bit casting materials, in canvas molding compounds, as fiberglass laminating resins and in non-metal machine-trunk fillers. They are also used as the thermoset polymer matrix in pre-pregs. Fiberglass-reinforced unsaturated polyesters find broad application in bodies of yachts and as body parts of cars.
Depending on the chemic structure, polyester can be a thermoplastic or thermoset. At that place are also polyester resins cured by hardeners; however, the well-nigh common polyesters are thermoplastics.[11] The OH group is reacted with an Isocyanate functional compound in a 2 component organization producing coatings which may optionally be pigmented. Polyesters as thermoplastics may modify shape subsequently the awarding of heat. While combustible at high temperatures, polyesters tend to shrink abroad from flames and self-extinguish upon ignition. Polyester fibers have high tenacity and E-modulus besides as low water absorption and minimal shrinkage in comparison with other industrial fibers.
Increasing the aromatic parts of polyesters increases their glass transition temperature, melting temperature, thermal stability, chemical stability, and solvent resistance.
Polyesters tin can also be telechelic oligomers like the polycaprolactone diol (PCL) and the polyethylene adipate diol (PEA). They are then used as prepolymers.
Aliphatic vs. aromatic polymers [edit]
Thermally stable polymers, which more often than not take a high proportion of effluvious structures, are as well chosen high-performance plastics. This application-oriented classification compares such polymers with technology plastics and commodity plastics. The continuous service temperature of loftier-performance plastics is generally stated as being college than 150 °C,[12] whereas engineering plastics (such as polyamide or polycarbonate) are oft divers as thermoplastics that retain their properties in a higher place 100 °C.[xiii] Commodity plastics (such equally polyethylene or polypropylene) have in this respect even greater limitations, but they are manufactured in bang-up amounts at low price.
Poly(ester imides) incorporate an aromatic imide group in the repeat unit, the imide-based polymers have a high proportion of aromatic structures in the main chain and vest to the class of thermally stable polymers. Such polymers contain structures that impart high melting temperatures, resistance to oxidative degradation and stability to radiation and chemical reagents. Amid the thermally stable polymers with commercial relevance are polyimides, polysulfones, polyetherketones, and polybenzimidazoles. Of these, polyimides are most widely applied.[14] The polymers' structures upshot likewise in poor processing characteristics, in detail a high melting indicate and low solubility. The named properties are in particular based on a high percentage of effluvious carbons in the polymer backbone which produces a certain stiffness.[xv] Approaches for an improvement of processability include the incorporation of flexible spacers into the backbone, the attachment of stable pendent groups or the incorporation of not-symmetrical structures.[xiv] Flexible spacers include, for example, ether or hexafluoroisopropylidene, carbonyl or aliphatic groups like isopropylidene; these groups allow bond rotation betwixt effluvious rings. Less symmetrical structures, for case based on meta- or ortho-linked monomers introduce structural disorder and thereby decrease the crystallinity.[iv]
The by and large poor processability of aromatic polymers (for example, a loftier melting signal and a low solubility) also limits the available options for synthesis and may crave stiff electron-altruistic co-solvents like HFIP or TFA for analysis (e. thou. iH NMR spectroscopy) which themselves tin can introduce further practical limitations.
Uses and applications [edit]
Fabrics woven or knitted from polyester thread or yarn are used extensively in dress and home furnishings, from shirts and pants to jackets and hats, bed sheets, blankets, upholstered article of furniture and computer mouse mats. Industrial polyester fibers, yarns and ropes are used in car tire reinforcements, fabrics for conveyor belts, safe belts, coated fabrics and plastic reinforcements with loftier-energy assimilation. Polyester cobweb is used as cushioning and insulating material in pillows, comforters and upholstery padding. Polyester fabrics are highly stain-resistant since polyester is a hydrophobic cloth, making it hard to absorb liquids. The only form of dyes which can be used to modify the color of polyester textile are what are known equally disperse dyes.[xvi]
Polyesters are besides used to brand bottles, films, tarpaulin, sails (Dacron), canoes, liquid crystal displays, holograms, filters, dielectric flick for capacitors, motion-picture show insulation for wire and insulating tapes. Polyesters are widely used as a finish on high-quality forest products such as guitars, pianos, and vehicle/yacht interiors. Thixotropic backdrop of spray-applicable polyesters make them ideal for utilize on open-grain timbers, as they can quickly fill wood grain, with a loftier-build picture show thickness per coat. It tin be used for fashionable dresses, merely it is most admired for its power to resist wrinkling and shrinking while washing the product. Its toughness makes information technology a frequent choice for children's vesture. Polyester is oft composite with other fibres like cotton wool to get the best of both worlds. Cured polyesters can be sanded and polished to a high-gloss, durable finish.
Production [edit]
Nuts [edit]
Polyethylene terephthalate, the polyester with the greatest market share, is a synthetic polymer made of purified terephthalic acid (PTA) or its dimethyl ester dimethyl terephthalate (DMT) and monoethylene glycol (Meg). With 18% market share of all plastic materials produced, it ranges 3rd afterward polyethylene (33.five%) and polypropylene (19.5%) and is counted equally article plastic.
In that location are several reasons for the importance of polyethylene terephthalate:
- The relatively piece of cake accessible raw materials PTA or DMT and 1000000
- The very well understood and described simple chemical process of its synthesis
- The low toxicity level of all raw materials and side products during production and processing
- The possibility to produce PET in a closed loop at depression emissions to the environment
- The outstanding mechanical and chemical properties
- The recyclability
- The broad variety of intermediate and last products.
In the following table, the estimated world polyester production is shown. Main applications are cloth polyester, bottle polyester resin, film polyester mainly for packaging and specialty polyesters for engineering plastics. According to this table, the world'due south total polyester production might exceed 50 1000000 tons per annum before the year 2010.
| Product type | 2002 (million tonnes/year) | 2008 (one thousand thousand tonnes/year) |
|---|---|---|
| Material-PET | xx | 39 |
| Resin, canteen/A-PET | 9 | 16 |
| Movie-PET | 1.2 | one.five |
| Special polyester | 1 | two.5 |
| Total | 31.ii | 59 |
Polyester processing [edit]
After the first stage of polymer production in the melt phase, the product stream divides into two dissimilar application areas which are mainly textile applications and packaging applications. In the following table, the main applications of textile and packaging of polyester are listed.
| Textile | Packaging |
|---|---|
| Staple cobweb (PSF) | Bottles for CSD, water, beer, juice, detergents, etc. |
| Filaments POY, DTY, FDY | A-PET film |
| Technical yarn and tire string | Thermoforming |
| Non-woven and spunbond | biaxial-oriented pic (BO-PET) |
| Mono-filament | Strapping |
Abbreviations:
- PSF
- Polyester-staple fiber;
- POY
- Partially oriented yarn;
- DTY
- Drawn textured yarn;
- FDY
- Fully fatigued yarn;
- CSD
- Carbonated soft drink;
- A-PET
- Amorphous polyethylene terephthalate film;
- BO-PET
- Biaxial-oriented polyethylene terephthalate movie;
A comparable small market place segment (much less than 1 1000000 tonnes/year) of polyester is used to produce engineering plastics and masterbatch.
In order to produce the polyester melt with a loftier efficiency, high-output processing steps like staple fiber (50–300 tonnes/day per spinning line) or POY /FDY (up to 600 tonnes/day separate into about x spinning machines) are meanwhile more than and more vertically integrated direct processes. This means the polymer melt is directly converted into the material fibers or filaments without the common footstep of pelletizing. We are talking well-nigh full vertical integration when polyester is produced at ane site starting from crude oil or distillation products in the chain oil → benzene → PX → PTA → PET cook → fiber/filament or bottle-grade resin. Such integrated processes are meanwhile established in more or less interrupted processes at ane production site. Eastman Chemicals were the commencement to innovate the idea of closing the chain from PX to PET resin with their so-called INTEGREX process. The capacity of such vertically integrated product sites is >grand tonnes/twenty-four hour period and can easily attain 2500 tonnes/day.
As well the in a higher place-mentioned large processing units to produce staple cobweb or yarns, there are ten thousands of small and very modest processing plants, so that one can gauge that polyester is candy and recycled in more than 10 000 plants around the globe. This is without counting all the companies involved in the supply industry, beginning with engineering science and processing machines and ending with special additives, stabilizers and colors. This is a gigantic industry complex and information technology is withal growing by four–viii% per year, depending on the world region.
Synthesis [edit]
Synthesis of polyesters is generally accomplished by a polycondensation reaction. The general equation for the reaction of a diol with a diacid is:
- (northward+one) R(OH)2 + n R'(COOH)two → HO[ROOCR'COO]northROH + 2n HtwoO.
Polyesters can be obtained past a wide range of reactions of which the most of import are the reaction of acids and alcohols, alcoholysis and or acidolysis of low-molecular weight esters or the alcoholysis of acyl chlorides. The following figure gives an overview over such typical polycondensation reactions for polyester production. Furthermore, polyesters are accessible via band-opening polymerization.

Azeotrope esterification is a classical method for condensation. The water formed by the reaction of booze and a carboxylic acid is continually removed by azeotrope distillation. When melting points of the monomers are sufficiently depression, a polyester can be formed via straight esterification while removing the reaction h2o via vacuum.

Directly bulk polyesterification at high temperatures (150 – 290 °C) is well-suited and used on the industrial scale for the production of aliphatic polyesters, unsaturated polyesters, and aromatic–aliphatic polyesters. Monomers containing phenolic or third hydroxyl groups exhibit a low reactivity with carboxylic acids and cannot be polymerized via direct acrid booze-based polyesterification.[4] In the example of PET production, however, the direct procedure has several advantages, in particular a college reaction rate, a higher attainable molecular weight, the release of water instead of methanol and lower storage costs of the acid when compared to the ester due to the lower weight.[ane]
Alcoholic transesterification [edit]

Transesterification: An booze-terminated oligomer and an ester-terminated oligomer condense to course an ester linkage, with loss of an alcohol. R and R' are the two oligomer bondage, R'' is a sacrificial unit such equally a methyl grouping (methanol is the byproduct of the esterification reaction).
The term "transesterification" is typically used to describe hydroxy–ester, carboxy–ester, and ester–ester exchange reactions. The hydroxy–ester substitution reaction possesses the highest charge per unit of reaction and is used for the production of numerous aromatic–aliphatic and wholly aromatic polyesters.[4] The transesterification based synthesis is particularly useful for when high melting and poorly soluble dicarboxylic acids are used. In improver, alcohols as condensation product are more volatile and thereby easier to remove than water.[17]
The high-temperature melt synthesis between bisphenol diacetates and aromatic dicarboxylic acids or in contrary between bisphenols and aromatic dicarboxylic acid diphenyl esters (carried out at 220 to 320 °C upon the release of acetic acid) is, as well the acyl chloride based synthesis, the preferred route to wholly aromatic polyesters.[4]
Acylation [edit]
In acylation, the acid begins as an acrid chloride, and thus the polycondensation proceeds with emission of muriatic acid (HCl) instead of water.
The reaction between diacyl chlorides and alcohols or phenolic compounds has been widely applied to polyester synthesis and has been subject of numerous reviews and book chapters.[iv][18] [19] [20] The reaction is carried out at lower temperatures than the equilibrium methods; possible types are the high-temperature solution condensation, amine catalysed and interfacial reactions. In addition, the use of activating agents is counted equally non-equilibrium method. The equilibrium constants for the acyl chloride-based condensation yielding yielding arylates and polyarylates are very high indeed and are reported to be 4.iii × 103 and 4.7 × 10three, respectively. This reaction is thus often referred to every bit a 'non-equilibrium' polyesterification. Even though the acyl chloride based synthesis is also subject of reports in the patent literature, it is unlikely that the reaction is utilized on the production scale.[21] The method is limited by the acrid dichlorides' high price, its sensitivity to hydrolysis and the occurrence of side reactions.[22]
The high temperature reaction (100 to > 300 °C) of an diacyl chloride with an dialcohol yields the polyester and hydrogen chloride. Under these relatively high temperatures the reaction proceeds rapidly without a catalyst:[20]
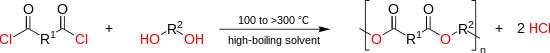
The conversion of the reaction tin can exist followed by titration of the evolved hydrogen chloride. A wide diversity of solvents has been described including chlorinated benzenes (e.g. dichlorobenzene), chlorinated naphthalenes or diphenyls, besides every bit non-chlorinated aromatics like terphenyls, benzophenones or dibenzylbenzenes. The reaction was besides practical successfully to the preparation of highly crystalline and poorly soluble polymers which require high temperatures to be kept in solution (at to the lowest degree until a sufficiently high molecular weight was achieved).[23]
In an interfacial acyl chloride-based reaction, the alcohol (generally in fact a phenol) is dissolved in the form of an alkoxide in an aqueous sodium hydroxide solution, the acyl chloride in an organic solvent immiscible with h2o such as dichloromethane, chlorobenzene or hexane, the reaction occurs at the interface nether high-speed agitation nearly room temperature.[20]

The procedure is used for the product of polyarylates (polyesters based on bisphenols), polyamides, polycarbonates, poly(thiocarbonate)southward, and others. Since the molecular weight of the product obtained past a high-temperature synthesis can be seriously express by side reactions, this trouble is circumvented past the mild temperatures of interfacial polycondensation. The procedure is applied to the commercial production of bisphenol-A-based polyarylates like Unitika'south U-Polymer.[iv] Water could exist in some cases replaced past an immiscible organic solvent (e. chiliad. in the adiponitrile/carbon tetrachloride system).[20] The procedure is of piddling use in the production of polyesters based on aliphatic diols which accept college pThousand a values than phenols and therefore practice not form alcoholate ions in aqueous solutions.[4] The base catalysed reaction of an acyl chloride with an alcohol may too be carried out in i phase using 3rd amines (due east. g. triethylamine, Et3Northward) or pyridine as acid acceptors:
![]()
While acyl chloride-based polyesterifications go along only very slowly at room temperature without a catalyst, the amine accelerates the reaction in several possible ways, although the machinery is not fully understood.[20] However, it is known that 3rd amines can crusade side-reactions such as the germination of ketenes and ketene dimers.[24]
- Silyl method
- In this variant of the HCl method, the carboxylic acid chloride is converted with the trimethyl silyl ether of the alcohol component and product of trimethyl silyl chloride is obtained
Acetate method (esterification) [edit]

- Silyl acetate method
Ring-opening polymerization [edit]

Aliphatic polyesters can be assembled from lactones under very mild conditions, catalyzed anionically, cationically, metallorganically or enzyme-based.[25] [26] A number of catalytic methods for the copolymerization of epoxides with cyclic anhydrides accept too recently been shown to provide a broad assortment of functionalized polyesters, both saturated and unsaturated. Ring-opening polymerization of lactones and lactides is also applied on the industrial scale.[27] [28]
Other methods [edit]
Numerous other reactions have been reported for the synthesis of selected polyesters, but are limited to laboratory-scale syntheses using specific weather condition, for example using dicarboxylic acid salts and dialkyl halides or reactions between bisketenes and diols.[4]
Instead of acyl chlorides, and then-chosen activating agents can be used, such as 1,i'-carbonyldiimidazole, dicyclohexylcarbodiimide, or trifluoroacetic anhydride. The polycondensation proceeds via the in situ conversion of the carboxylic acrid into a more reactive intermediate while the activating agents are consumed. The reaction proceeds, for example, via an intermediate N-acylimidazole which reacts with catalytically acting sodium alkoxide:[4]

The use of activating agents for the production of high-melting aromatic polyesters and polyamides under mild weather has been field of study of intensive academic enquiry since the 1980s, just the reactions have not gained commercial credence as similar results can exist achieved with cheaper reactants.[4]
Thermodynamics of polycondensation reactions [edit]
Polyesterifications are grouped by some authors[four] [18] into two principal categories: a) equilibrium polyesterifications (mainly alcohol-acrid reaction, alcohol–ester and acid–ester interchange reactions, carried out in majority at high temperatures), and b) not-equilibrium polyesterifications, using highly reactive monomers (for example acid chlorides or activated carboxylic acids, mostly carried out at lower temperatures in solution).
The acid-booze based polyesterification is one example of an equilibrium reaction. The ratio between the polymer-forming ester group (-C(O)O-) and the condensation production water (HtwoO) against the acid-based (-C(O)OH) and alcohol-based (-OH) monomers is described past the equilibrium constant 1000C .
The equilibrium constant of the acid-alcohol based polyesterification is typically ThouC ≤ 10, what is not loftier enough to obtain loftier-molecular weight polymers (DPn ≥ 100), equally the number boilerplate degree of polymerization (DPn ) can be calculated from the equilibrium constant YardC .[19]
In equilibrium reactions, it is therefore necessary to remove the condensation production continuously and efficiently from the reaction medium in order to drive the equilibrium towards polymer.[nineteen] The condensation product is therefore removed at reduced pressure and high temperatures (150–320 °C, depending on the monomers) to prevent the back reaction.[eight] With the progress of the reaction, the concentration of active chain ends is decreasing and the viscosity of the melt or solution increasing. For an increase of the reaction charge per unit, the reaction is carried out at high cease grouping concentration (preferably in the bulk), promoted by the elevated temperatures.
Equilibrium constants of magnitude GrandC ≥ 10iv are achieved when using reactive reactants (acid chlorides or acid anhydrides) or activating agents like i,1′-carbonyldiimidazole. Using these reactants, molecular weights required for technical applications can be accomplished even without active removal of the condensation product.
History [edit]
In 1926, United States-based Due east.I. du Pont de Nemours and Co. began research on large molecules and synthetic fibers. This early on research, headed by W.H. Carothers, centered on what became nylon, which was one of the starting time synthetic fibers.[29] Carothers was working for duPont at the time. Carothers' research was incomplete and had not avant-garde to investigating the polyester formed from mixing ethylene glycol and terephthalic acrid. In 1928 polyester was patented in United kingdom of great britain and northern ireland by the International General Electric company.[xxx] Carothers' projection was revived by British scientists Whinfield and Dickson, who patented polyethylene terephthalate (PET) or PETE in 1941. Polyethylene terephthalate forms the basis for constructed fibers like Dacron, Terylene and polyester. In 1946, duPont bought all legal rights from Regal Chemical Industries (ICI).[one]
Biodegradation and environmental concerns [edit]
The Futuro houses were fabricated of fibreglass-reinforced polyester plastic; polyester-polyurethane, and poly(methyl methacrylate). One house was found to exist degrading by cyanobacteria and Archaea.[31] [32]
Cantankerous-linking [edit]
Unsaturated polyesters are thermosetting polymers. They are by and large copolymers prepared by polymerizing one or more diols with saturated and unsaturated dicarboxylic acids (maleic acid, fumaric acid, etc.) or their anhydrides. The double bond of unsaturated polyesters reacts with a vinyl monomer, unremarkably styrene, resulting in a 3-D cross-linked structure. This structure acts equally a thermoset. The exothermic cross-linking reaction is initiated through a catalyst, usually an organic peroxide such every bit methyl ethyl ketone peroxide or benzoyl peroxide.
Pollution of freshwater and seawater habitats [edit]
A squad at Plymouth Academy in the UK spent 12 months analysing what happened when a number of constructed materials were washed at different temperatures in domestic washing machines, using different combinations of detergents, to quantify the microfibres shed. They found that an average washing load of six kg could release an estimated 137,951 fibres from polyester-cotton fiber blend fabric, 496,030 fibres from polyester and 728,789 from acrylic. Those fibers add together to the general microplastics pollution.[33] [34] [35]
Encounter likewise [edit]
- Epoxy
- Glycerine phthalate
- Microfiber
- Oligoester
- Polyamide
- Rayon
- Viscose
References [edit]
- ^ a b c d east Köpnick H, Schmidt Grand, Brügging W, Rüter J, Kaminsky W (June 2000). "Polyesters". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim, Germany: Wiley-VCH Verlag GmbH & Co. KGaA.
- ^ Mendelson C (17 May 2005). Domicile Comforts: The Fine art and Science of Keeping Business firm. Simon and Schuster. ISBN9780743272865.
- ^ "Thermal Spray Abradable Coatings". www.gordonengland.co.united kingdom . Retrieved 12 Dec 2018.
- ^ a b c d e f g h i j k fifty Rogers ME, Long TE (2003). Synthetic Methods in Stride-Growth Polymers. Hoboken, NJ, USA: John Wiley & Sons, Inc.
- ^ Hefetz, A., et al. (1979). Natural polyesters: Dufour's gland macrocyclic lactones form breed cell laminesters in Colletes bees. Science 204(4391), 415-17.
- ^ Eveleth, R. and D. Chachra. Tin Bees Brand Tupperware? Scientific American December xix, 2011.
- ^ Kong Ten, Qi H, Curtis JM (August 2014). "Synthesis and label of high-molecular weight aliphatic polyesters from monomers derived from renewable resources". Periodical of Applied Polymer Scientific discipline. 131 (15): 40579–40586. doi:x.1002/app.40579.
- ^ a b Park HS, Seo JA, Lee HY, Kim HW, Wall IB, Gong MS, Knowles JC (August 2012). "Synthesis of rubberband biodegradable polyesters of ethylene glycol and butylene glycol from sebacic acid". Acta Biomaterialia. viii (viii): 2911–eight. doi:10.1016/j.actbio.2012.04.026. PMID 22522011.
- ^ Gurunathan T, Mohanty S, Nayak SK (January 2016). "Hyperbranched polymers for coating applications: a review". Polymer-Plastics Engineering and Engineering. 55 (i): 92–117. doi:ten.1080/03602559.2015.1021482. S2CID 100936296.
- ^ Testud B, Pintori D, Grau East, Taton D, Cramail H (2017). "Hyperbranched polyesters by polycondensation of fatty acid-based AB northward-blazon monomers". Greenish Chemistry. xix (1): 259–69. arXiv:1911.07737. doi:10.1039/C6GC02294D. S2CID 102450135.
- ^ Rosato DV, Rosato DV, Rosato MV (2004). Plastic product cloth and procedure option handbook. Elsevier. p. 85. ISBN978-1-85617-431-2.
- ^ Parker, David; Bussink, Jan; van de Grampel, Hendrik T.; Wheatley, Gary Due west.; Dorf, Ernst-Ulrich; Ostlinning, Edgar; Reinking, Klaus; Schubert, Frank; Jünger, Oliver (xv Apr 2012), "Polymers, Loftier-Temperature", in Wiley-VCH Verlag GmbH & Co. KGaA (ed.), Ullmann's Encyclopedia of Industrial Chemical science, Weinheim, Germany: Wiley-VCH Verlag GmbH & Co. KGaA, pp. a21_449.pub3, doi:10.1002/14356007.a21_449.pub4, ISBN978-3-527-30673-ii , retrieved xiii December 2020
- ^ H.-G. Elias and R. Mülhaupt, in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Frg, 2015, pp. 1–70.
- ^ a b P. Eastward. Cassidy, T. M. Aminabhavi and 5. Southward. Reddy, in Kirk-Othmer Encyclopedia of Chemical Engineering, John Wiley & Sons, Inc., Hoboken, NJ, Us, 2000.
- ^ T. Whelan, Polymer Applied science Dictionary, Springer Netherlands, Dordrecht, 1994.
- ^ Schuler MJ (1981). "Part viii: Dyeing with disperse dyes". Dyeing Primer. AATCC. p. 21. GGKEY:SK3T00EYAFR.
- ^ Ravve A (2012). Principles of Polymer Chemistry. New York, New York, NY: Springer.
- ^ a b Vinogradova SV (January 1977). "The bones principles of non-equilibrium polycondensation". Polymer Science USSR. nineteen (4): 769–808. doi:ten.1016/0032-3950(77)90232-5.
- ^ a b c Duda A, Penczek S (2005). "Mechanisms of Aliphatic Polyester Formation". In Doi Y, Steinbüchel A (eds.). Biopolymers Online. Weinheim, Frg: Wiley-VCH Verlag GmbH & Co. KGaA. pp. 371–383. doi:ten.1002/3527600035.bpol3b12.
- ^ a b c d eastward Pilati F (1989). "Polyesters". Comprehensive Polymer Scientific discipline and Supplements. Vol. 5. Elsevier. pp. 275–315.
- ^ Lienert KW (1999). "Poly (ester-imide)southward for industrial use.". In Kricheldorf 60 minutes (ed.). Progress in Polyimide Chemistry II. Advances in Polymer Science. Vol. 141. Berlin, Heidelberg: Springer. pp. 45–82. doi:10.1007/3-540-49814-1_2. ISBN978-iii-540-64963-2.
- ^ Sokolsky-Papkov K, Langer R, Domb AJ (Apr 2011). "Synthesis of aliphatic polyesters by polycondensation using inorganic acid as catalyst". Polymers for Advanced Technologies. 22 (v): 502–511. doi:x.1002/pat.1541. PMC4249767. PMID 25473252.
- ^ Sokolsky-Papkov M, Langer R, Domb AJ (Apr 2011). "Synthesis of aliphatic polyesters by polycondensation using inorganic acid as catalyst". Polymers for Avant-garde Technologies. 22 (five): 502–511. doi:ten.1002/pat.1541. PMC4249767. PMID 25473252.
- ^ Kricheldorf HR, Nuyken O, Swift Grand (2004). Handbook of Polyermer Synthesis (2nd ed.). CRC Press. ISBN0-367-57822-0. OCLC 1156408945.
- ^ Varma IK, Albertsson Ac, Rajkhowa R, Srivastava RK (October 2005). "Enzyme catalyzed synthesis of polyesters". Progress in Polymer Science. thirty (x): 949–81. doi:10.1016/j.progpolymsci.2005.06.010.
- ^ Nuyken O, Pask SD (April 2013). "Ring-Opening Polymerization—An Introductory Review". Polymers. five (ii): 361–403. doi:10.3390/polym5020361. ISSN 2073-4360.
- ^ Jérôme C, Lecomte P (June 2008). "Contempo advances in the synthesis of aliphatic polyesters by band-opening polymerization". Advanced Drug Delivery Reviews. 60 (9): 1056–76. doi:10.1016/j.addr.2008.02.008. PMID 18403043.
- ^ Dechy-Cabaret O, Martin-Vaca B, Bourissou D (December 2004). "Controlled band-opening polymerization of lactide and glycolide". Chemic Reviews. 104 (12): 6147–76. doi:x.1021/cr040002s. PMID 15584698.
- ^ "How polyester is made - material, manufacture, making, history, used, construction, steps, product, History". www.madehow.com . Retrieved 4 Dec 2018.
- ^ Loasby Thou (1951). "The Development of the Constructed Fibres". Journal of the Textile Found Proceedings. 42 (8): P411–P441. doi:10.1080/19447015108663852.
- ^ Cappitelli F, Principi P, Sorlini C (August 2006). "Biodeterioration of mod materials in contemporary collections: can biotechnology help?". Trends in Biotechnology. 24 (8): 350–iv. doi:10.1016/j.tibtech.2006.06.001. PMID 16782219.
- ^ Rinaldi A (November 2006). "Saving a fragile legacy. Biotechnology and microbiology are increasingly used to preserve and restore the world'south cultural heritage". EMBO Reports. vii (11): 1075–ix. doi:10.1038/sj.embor.7400844. PMC1679785. PMID 17077862.
- ^ O'Connor MC (27 October 2014). "Inside the lonely fight against the biggest environmental problem you've never heard of". The Guardian.
- ^ Williams A. "Washing clothes releases thousands of microplastic particles into environment, study shows". Plymouth University. Retrieved 9 October 2016.
- ^ Napper IE, Thompson RC (November 2016). "Release of synthetic microplastic plastic fibres from domestic washing machines: Furnishings of fabric blazon and washing weather condition". Marine Pollution Bulletin. 112 (1–2): 39–45. doi:10.1016/j.marpolbul.2016.09.025. hdl:10026.1/8163. PMID 27686821.
Further reading [edit]
- Textiles, by Sara Kadolph and Anna Langford. 8th Edition, 1998.
External links [edit]
- Lipase catalyzed polyesterification: Enzyme-Catalyzed Polymerization of Terminate-Functionalized Polymers in a Microreactor
Source: https://en.wikipedia.org/wiki/Polyester
Posted by: bockmartyart49.blogspot.com

![{\displaystyle K_{C}={\frac {[...-C(O)O-...][H_{2}O]}{[-C(O)OH][-OH]}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/cbecd6b9db291421d8e8aabedb5fc032e5f9b3f0)
![{\displaystyle DP_{n}~=~{\sqrt[{2}]{K_{C}}}+1}](https://wikimedia.org/api/rest_v1/media/math/render/svg/5299262cb76bec69c3a62c8dab17c3cc904da956)
0 Response to "What Is The Differences Between The Makeup And Structure Of Salt And Polyester?"
Post a Comment